The pH of Your Blood
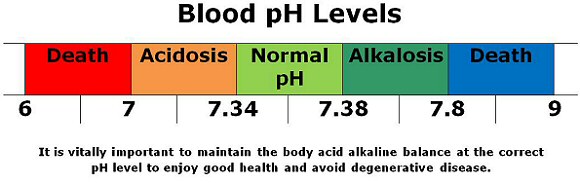
The pH of your blood is tightly regulated by a complex system of buffers that are continuously at work to maintain a range of 7.35 to 7.45. If the pH of your blood falls below 7.35, the result is a condition called acidosis, a state that leads to central nervous system depression. Severe acidosis – where blood pH falls below 7.00 – can lead to a coma and even death. If the pH of your blood rises above 7.45, the result is alkalosis. Severe alkalosis can also lead to death, but through a different mechanism; alkalosis causes all of the nerves in your body to become hypersensitive and over-excitable, often resulting in muscle spasms, nervousness, and convulsions; it’s usually the convulsions that cause death in severe cases.
When excess acidity enters your body, the buffering system will regulate the blood’s pH level by borrowing minerals from vital organs. Minerals including calcium, sodium, potassium and magnesium. At first, excess acidity may not show visible signs for a few years, but after borrowing large amounts of minerals from your vital organs, it will weaken your body and you will notice your health deteriorate faster as you age.
Even though you are generally healthy and do not have any illnesses and diseases, you will want to maintain an alkalizing diet and lifestyle. If you neglect your diet and lifestyle now, you might be highly at risk to deadly diseases and even cancer that may only surface many years later. Having an acidic body will cause an increase in free radicals in your body. Free radicals are atoms or groups of atoms with an odd (unpaired) number of electrons and can be formed when oxygen interacts with certain molecules. Cells may function poorly or die if this occurs. Free radicals are also molecules responsible for aging and tissue damage.
A simple urine test measures how acidic your urine is. Blood pH is an overall composite of what is going on in the whole body and is regulated primarily by your kidneys, lungs, intestines, and liver. To know exactly what is going on at the cellular level would be ideal, but it is not clinically practical to measure the pH within the cell. The most useful pH measure for following one’s diet and consumption of acid-producing or alkalinizing foods is to measure urine pH. Urine pH provides you with rapid feedback regarding the nature of your diet. A rapid change in urine pH does not signify that a rapid change in blood pH will follow. However, if urine pH is constantly measured as acidic over long periods of time (months), it is indicative that the body is chronically producing large amounts of acid.
Our diet might be deprived of the essential nutrients which is important to alkalize your body and lose weight naturally. By providing a clean environment for your body cells, you are giving them the very best protection against becoming diseased, and remember, all illness starts at the cellular level. A good approximation of tissue pH is easily obtained by testing the pH of your saliva or first-morning urine. These tests are available at your local health food stores or online with complete instructions on how to use them.